Abstract
BACKGROUND: Impact of Plerixafor (P) mobilized stem cells on immune reconstitution of autologous stem cell transplant (ASCT) patients has not been established. Early lymphocyte recovery (Absolute lymphocyte count of > 1 K/uL at day 30 after transplant) has been established to predict outcomes in multiple myeloma (MM) patients. The purpose of this study was to evaluate lymphocyte recovery in MM patients who underwent ASCT with stem cells mobilized with G-CSF vs G-CSF+P. Secondary objective was to evaluate the survival outcomes.
METHODS: This is a retrospective analysis of MM patients who underwent first ASCT between 2008 and 2016 with either G-CSF or G-CSF+P mobilization at Karmanos Cancer Institute in Detroit, Michigan. Plerixafor was used per institutional guidelines when the peripheral CD-34+ve cell-count was < 20/ uL on day 5 of G-CSF mobilization. 610 total patients were identified. Mobilization agents used were G-CSF alone (n= 469) or G-CSF+P (n= 141). All patients underwent transplant after Melphalan (M) conditioning and dose of M was at treating physician's discretion (140 vs 200 mg/m2). The primary endpoint was the Absolute Lymphocyte Count at day 30 (ALC30). Secondary endpoints were PFS and OS Univariable and multivariable Cox proportional hazards regression models were fit to assess associations between ten pre chosen predictors( age, race, stage at diagnosis, doublet vs. triplet therapy, lines of treatment, disease status, mobilization agents, melphalan dose, ALC at day 30, post- transplant maintenance) and survival benefit (PFS and OS).
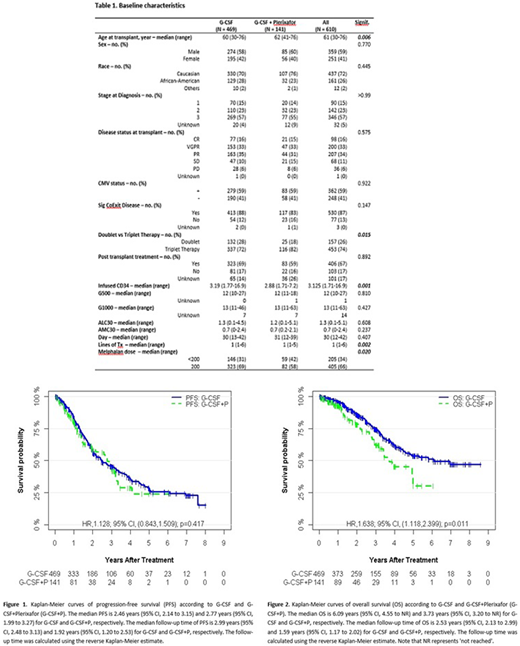
RESULTS: Median age of patients was older in G-CSF+P group (62 vs 60 years, p=. 006) and they were more likely to receive triplet therapy (82 vs 72%, p=. 015) for induction compared to G-CSF group (Table 1). Patients in G-CSF group were more likely to receive greater than one line of treatment before transplant (p=. 006). Disease status at transplant was similar between the two groups. G-CSF patients received higher dose of M (at 200mg/ m2) more frequently (69 vs. 58%, p = 0.010) and median cell dose infused was higher in G-CSF group (3.19 vs 2.88 x106 CD 34+ve-cells/Kg, p= 0.001).
Primary endpoint, ALC30, was 1.3 K/uL(.1-4.5) and 1.2 K/uL(.1-5.1) for G-CSF and G-CSF+P, respectively (p=. 608). Median day to neutrophil recovery were similar in both groups (ANC of 500 at Day 12). Post-transplant maintenance use was similar between the two groups. The median PFS was 2.46 years (95% CI, 2.14 to 3.15) and 2.77 years (95% CI, 1.99 to 3.27) for G-CSF and G-CSF+P, respectively (HR: 1.128; 95% CI, (.843-1.509); p=. 417) (Figure 1). The median OS was 6.09 years (95% CI, 4.55 to NR) and 3.73 years (95% CI, 3.20 to NR) for G-CSF and G-CSF+P, respectively (HR: 1.638; 95% CI, (1.118-2.399); p=. 011) (Figure 2) .In MVA, higher stage at diagnosis, less than PR before ASCT, and no post-transplant maintenance therapy were associated with worse PFS and OS. More lines of treatment adversely impacted PFS. Use of G-CSF+P for mobilization and Melphalan dose ≤ 200-mg/ m2 adversely impacted OS. ALC30 did not impact PFS or OS in MVA. There were no significant differences in causes of death among the 2 groups.
CONCLUSIONS: In this large, retrospective analysis of MM patients mobilized with G-CSF vs G-CSF + P, there was no significant difference in lymphocyte recovery. Higher Melphalan dose resulted in improved OS in the MVA. There was an overall survival difference favoring the G-CSF group, however, differences in baseline characteristics not accounted for in the MVA may be responsible for this observation. A Prospective study comparing these mobilization regimens including patients with similar baseline characteristics is necessary to confirm this finding.
Deol:Kite Pharmaceuticals: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.